Your Location:Home > Products > Chemical Reagents > methyl 4-piperidinecarboxylate


CasNo: 2971-79-1
MF: C7H13NO2
Appearance: White to off-white crysrallic powder
Methyl 4-piperidinecarboxylate is a colorless to pale yellow liquid that serves as an important intermediate in organic synthesis. As a derivative of piperidine characterized by a carboxylic acid ester group attached to the fourth carbon of the piperidine ring, it typically appears as a clear liquid. This compound is synthesized through specific chemical reactions, such as the esterification of 4-piperidinecarboxylic acid with methanol under suitable conditions. Methyl 4-piperidinecarboxylate finds applications in drug development, pesticide synthesis, and materials science. In drug synthesis, it may act as a key structural unit for molecules with specific pharmacological activity, particularly those targeting the central nervous system. Additionally, it plays a role in the production of agrochemicals and other specialized chemicals, making it a versatile component in various industries.
InChI:InChI=1/C7H13NO2/c1-10-7(9)6-2-4-8-5-3-6/h6,8H,2-5H2,1H3
To obtain selective and potent inhibitor for T-type calcium channel by ligand based drug design, 4-piperidinecarboxylate and 4-piperidinecyanide derivatives were prepared and evaluated for in vitro and in vivo activity against α1G calcium channel. Among them, several compounds showed good T-type calcium channel inhibitory activity and minimal off-target activity over hERG channel (% inhibition at 10 μM = 61.85–71.99, hERG channel IC50 = 1.57 ± 0.14–4.98 ± 0.36 μM). Selected compound 31a was evaluated on SNL model of neuropathic pain and showed inhibitory effect on mechanical allodynia.
A study of the stereo- and face selectiv...
Currently, synergistic inhibition of pol...
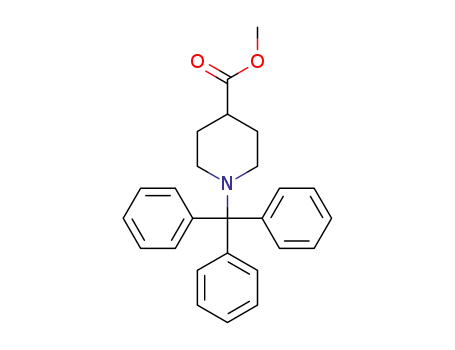
N-tritylisonipecotic acid methyl ester

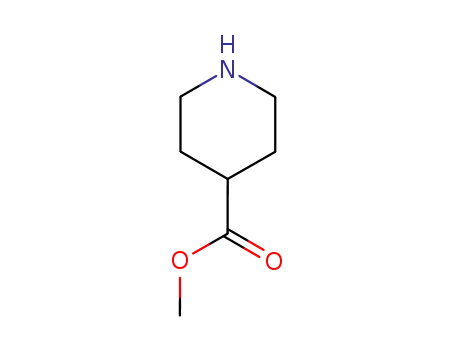
isonipecotic acid methyl ester
| Conditions | Yield |
|---|---|
|
With ammonium cerium (IV) nitrate; water; acetic acid; In dichloromethane; at 20 ℃; for 1h; Inert atmosphere;
|
89% |
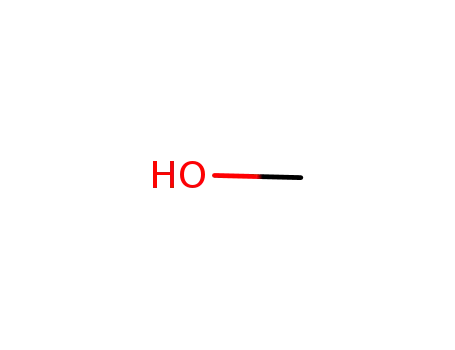
methanol

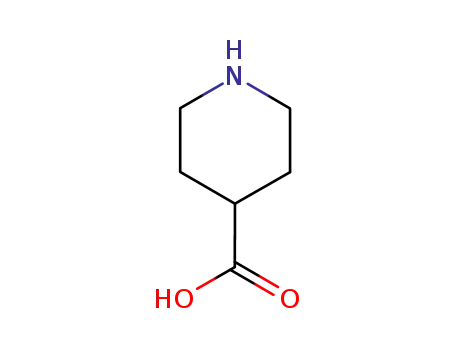
isonipecotic acid


isonipecotic acid methyl ester
| Conditions | Yield |
|---|---|
|
With thionyl chloride; at 0 ℃; Reflux;
|
99% |
|
With hydrogenchloride; for 4h;
|
70% |
|
With thionyl chloride;
|
|
|
With thionyl chloride; for 1h; Heating;
|
|
|
With hydrogenchloride; for 3h; Heating;
|
|
|
With thionyl chloride; at 20 ℃;
|
|
|
With thionyl chloride; at 0 - 20 ℃; for 18h;
|
|
|
With thionyl chloride; at 0 - 20 ℃; for 18h;
|
|
|
With thionyl chloride; at 0 - 20 ℃; for 18h;
|
|
|
With sulfuryl dichloride; at 20 - 50 ℃;
|
|
|
With thionyl chloride; at 0 - 20 ℃; for 18h;
|
|
|
With thionyl chloride; In methanol; at 0 - 25 ℃; for 12h;
|
|
|
With thionyl chloride; at 0 ℃; for 1h; Inert atmosphere; Reflux;
|

isonipecotic acid
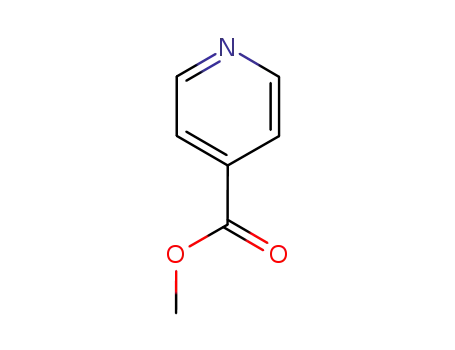
4-pyridinecarboxylic acid, methyl ester

methanol
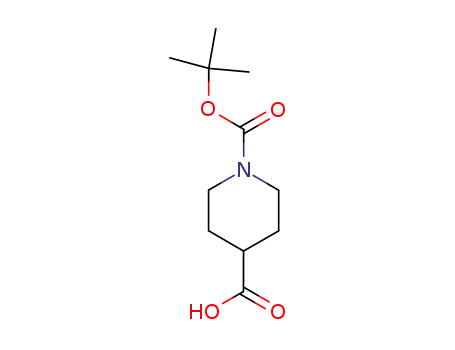
N-[(tert-butoxy)carbonyl]piperidine-4-carboxylic acid
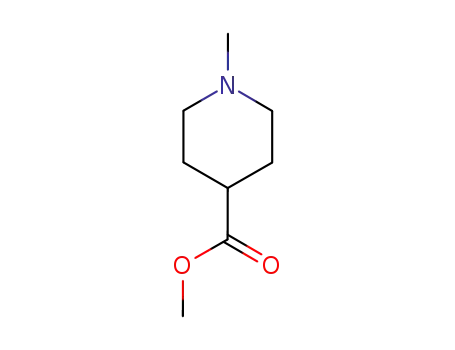
1-methyl-piperidine-4-carboxylic acid methyl ester
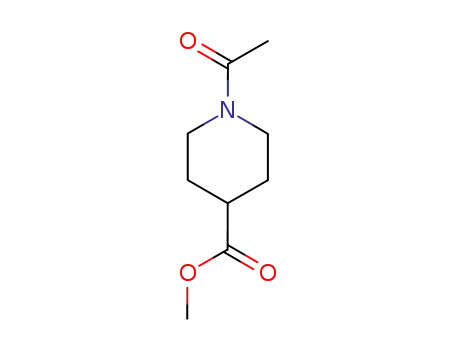
1-acetyl-piperidine-4-carboxylic acid methyl ester
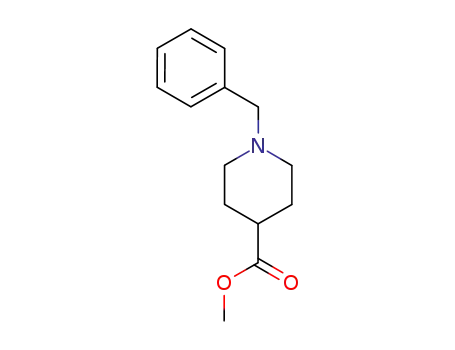
1-benzylpiperidine-4-carboxylic acid methyl ester