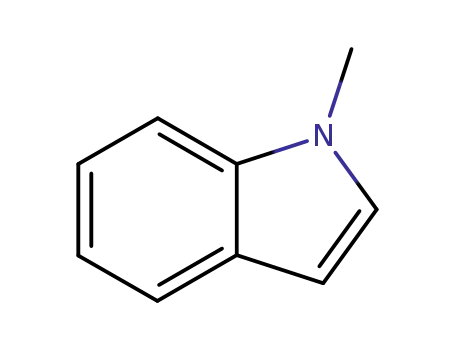
Factory Supply industrial standard 5-Methoxyindole 1006-94-6 In Stock
- Molecular Formula:C9H9NO
- Molecular Weight:147.177
- Appearance/Colour:white to light brownish crystalline powder
- Vapor Pressure:0.00234mmHg at 25°C
- Melting Point:52-55 °C(lit.)
- Refractive Index:1.637
- Boiling Point:311.9 °C at 760 mmHg
- PKA:16.70±0.30(Predicted)
- Flash Point:109.2 °C
- PSA:25.02000
- Density:1.169 g/cm3
- LogP:2.17650
5-Methoxyindole(Cas 1006-94-6) Usage
5-Methoxyindole is a white to light yellow crystalline powder that plays a significant role in both organic and medicinal chemistry. As a derivative of indole characterized by a methoxy group (-OCH3) attached to the fifth carbon atom of the indole ring, it typically appears as a solid at room temperature. This compound serves as a key building block in the synthesis of numerous natural products and pharmaceuticals and exhibits potential biological activity. Synthesized through specific chemical reactions, such as the reaction of indole with methanol in the presence of an appropriate catalyst, 5-methoxyindole is often used as an intermediate in the production of various pharmaceuticals and agrochemicals. In drug development, it may contribute to the construction of compounds with antidepressant, anti-inflammatory, or anti-tumor activities. For instance, the development of some new antidepressant drugs may utilize the unique structural characteristics of 5-methoxyindole. Additionally, due to its structural features, 5-methoxyindole can be involved in the creation of drugs targeting specific receptors or enzymes and may find applications in the production of dyes and pigments, depending on ongoing research and industrial needs.
InChI:InChI=1/C9H9NO/c1-11-8-3-2-7-4-5-10-9(7)6-8/h2-6,10H,1H3
1006-94-6 Relevant articles
5-methoxyindole metabolites of L-tryptophan: control of COX-2 expression, inflammation and tumorigenesis
Kenneth K Wu, Huei-Hsuan Cheng & Tzu-Ching Chang
, Journal of Biomedical Science, Volume 21, article number 17, (2014)
The physiological relevance of 5-MTP as an endogenous regulator of inflammation and cancer metastasis remains to be investigated. On the other hand, 5-methoxyindole metabolites of tryptophan are valuable lead compounds for development of new anti-inflammatory drugs and cancer chemoprevention.
5-methoxyindole metabolites of L-tryptophan: control of COX-2 expression, inflammation and tumorigenesis
Elavarasan, Samaraj,Bhaumik, Asim,Sasidharan, Manickam
, Journal of Biomedical Science, Volume 21, article number 17, (2014)
Cyclooxygenase-2(COX-2) overexpression promotes inflammation and tumorigenesis. COX-2 expression in response to diverse stimuli is tightly controlled to avoid persistent overexpression. 5-methoxyindole metabolites of L-tryptophan represent a new class of compounds that control COX-2 expression at the transcriptional level. Two of the metabolites, the newly discovered 5-methoxytryptophan (5-MTP, also known as cytoguardin) and N-acetyl 5-methoxytryptamine (melatonin) are the focus of this review.
1006-94-6 Process route
-
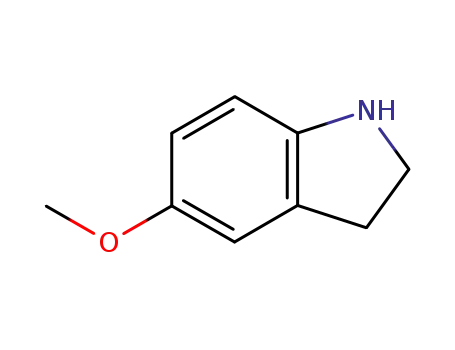
- 21857-45-4
5-methoxyindoline
-
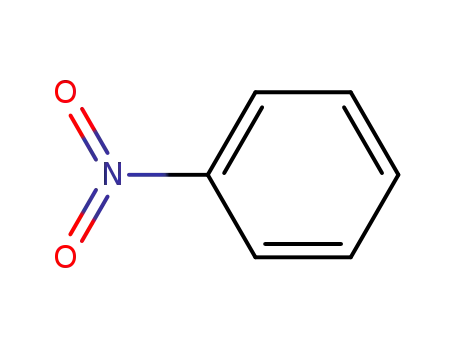
- 98-95-3,26969-40-4
nitrobenzene
-
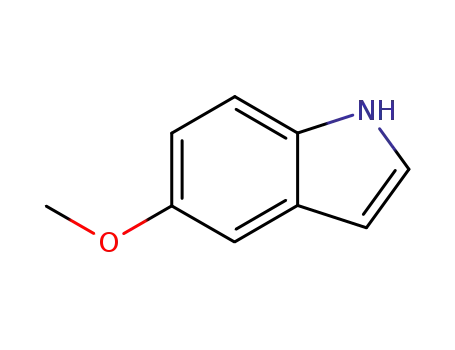
- 1006-94-6
5-methoxylindole
Conditions
| Conditions |
Yield |
|
With nickel-nitrogen-doped carbon framework; In water; at 145 ℃; for 18h; Inert atmosphere; Sealed tube; Green chemistry;
|
71%
68% |
-
- 94493-20-6
tetramethylviolacein
-
- 1006-94-6
5-methoxylindole
-
- 61-70-1
N-methyl-2-indolinone
-
- 2521-13-3
5-methoxy-N-methylindole
Conditions
| Conditions |
Yield |
|
With zinc; Product distribution; pyrolyses;
|
|
1006-94-6 Upstream products
-
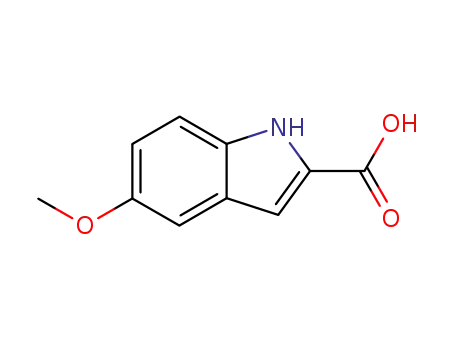
4382-54-1
5-methoxy-1H-indole-2-carboxylic acid
-
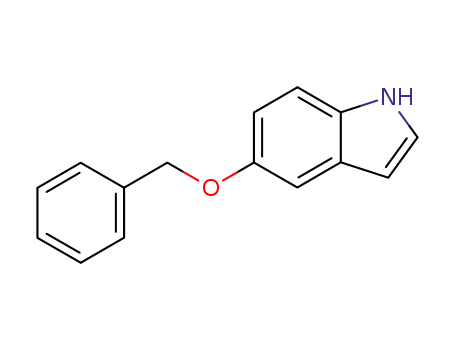
1215-59-4
5-benzyloxy-1H-indole
-
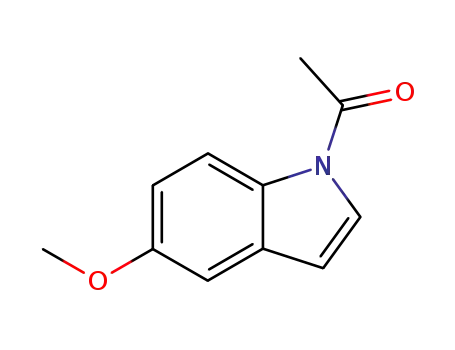
58246-80-3
1-(5-methoxy-1H-indol-1-yl)ethan-1-one
-
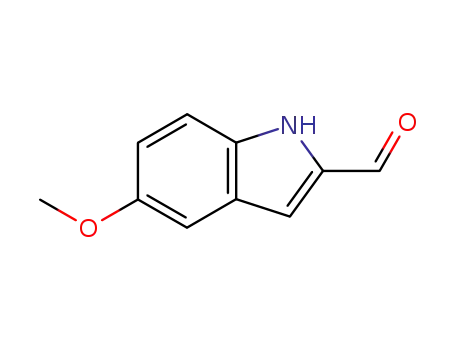
21778-81-4
5-methoxy-1H-indole-2-carbaldehyde
1006-94-6 Downstream products
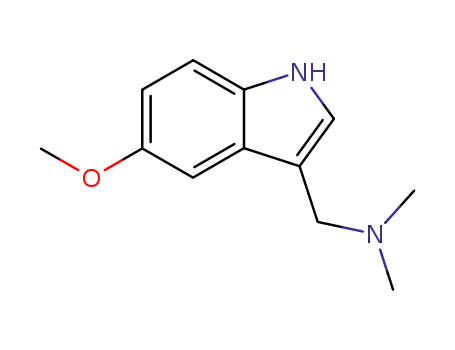
-
16620-52-3
5-methoxygramine
-
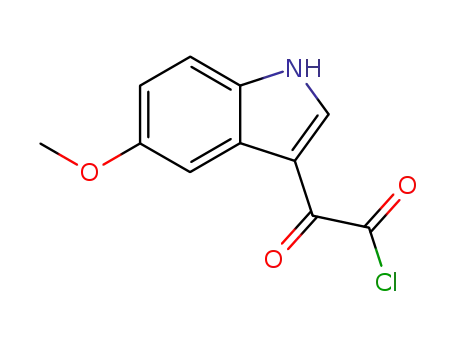
2426-19-9
(5-methoxy-1H-indol-3-yl)oxoacetyl chloride
-
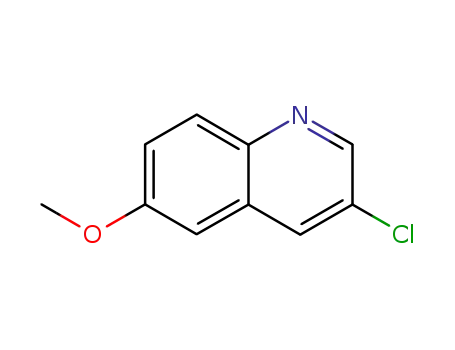
861553-63-1
3-chloro-6-methoxy-quinoline
-
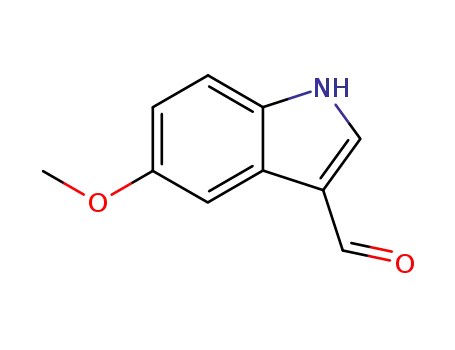
10601-19-1
5-methoxyindole-3-carboxaldehyde