Your Location:Home > Products > Chemical Reagents > D-CAMPHOR


CasNo: 464-49-3
MF: C10H16O
Appearance: white crystals
D-Camphor is an organic compound with a special smell and properties. In the field of medicine, it has certain medicinal value, such as stimulating local blood circulation and relieving pain, and is often used in some external medicines. In industry, D-Camphor can be used to make products such as spices and insect repellents. Its volatility and unique smell make it play a role in insect repellent and deodorization. However, it should be noted that D-Camphor has a certain toxicity and should be used in accordance with relevant regulations and safety guidelines. D-Camphor is generally extracted from plants of the Lauraceae family or prepared by chemical synthesis.
InChI:InChI=1/C10H16O/c1-9(2)7-4-5-10(9,3)8(11)6-7/h7H,4-6H2,1-3H3/t7?,10-/m0/s1
Enantioselective desymmetrization of cyclohexadienones via a D-camphor-derived triazolium salt catalyzed intramolecular Stetter reaction was realized. With 10 mol% of camphor-derived triazolium salt E and 10 mol% of DIEA, various substituted cyclohexadienones proceeded through an intramolecular Stetter reaction, affording tricyclic products in moderate to good yields and excellent ee.
A green route to oxidize terpenic alcoho...
The development of sustainable processes...
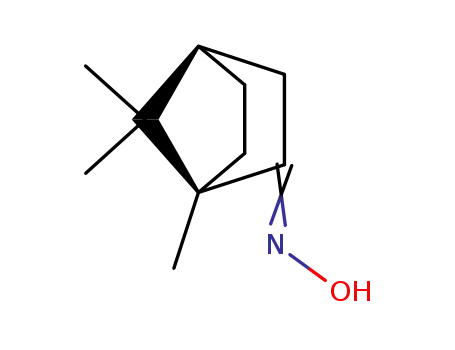
(R,R)-camphor oxime

![(1R,4R)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one](/upload/2024/7/84c09009-3624-4cb7-b203-eaa2cc40decc.png)
(1R,4R)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one

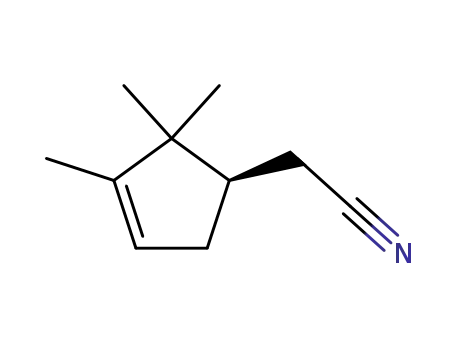
((R)-2,2,3-Trimethyl-cyclopent-3-enyl)-acetonitrile

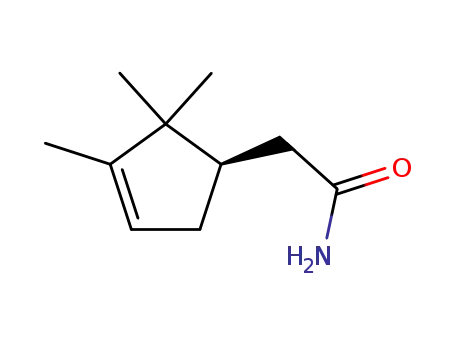
α-campholenic amide

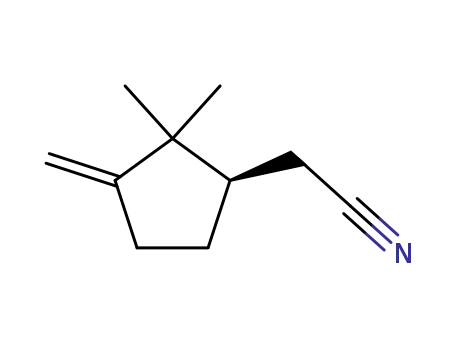
2,2-dimethyl-3-methylenecyclopentylacetonitrile
| Conditions | Yield |
|---|---|
|
In methanol; at 20 - 30 ℃; for 15h; Further byproducts given. Yields of byproduct given; Irradiation;
|
32% |

(R,R)-camphor oxime

![(1R,4R)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one](/upload/2024/7/84c09009-3624-4cb7-b203-eaa2cc40decc.png)
(1R,4R)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one


((R)-2,2,3-Trimethyl-cyclopent-3-enyl)-acetonitrile


2,2-dimethyl-3-methylenecyclopentylacetonitrile

1,8,8-trimethyl-2-azabicyclo<3.2.1>octan-3-one
| Conditions | Yield |
|---|---|
|
In methanol; at 20 - 30 ℃; for 15h; Further byproducts given. Yields of byproduct given; Irradiation;
|
12% |
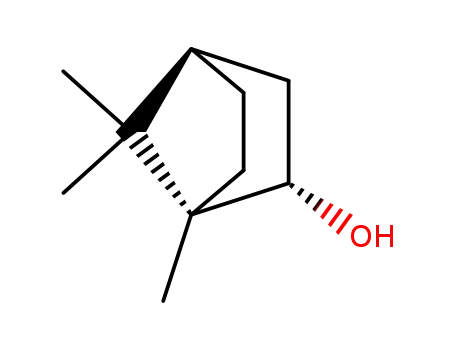
(+)-borneol
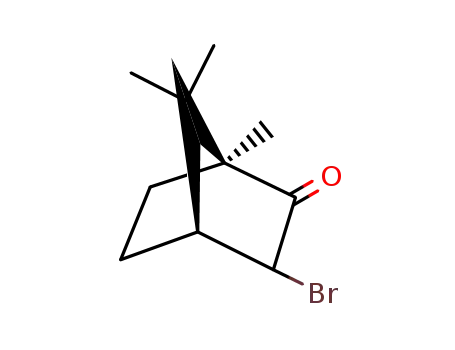
(1S,4S)-3-bromo-1,7,7-trimethylbicyclo[2.2.1]heptan-2-one
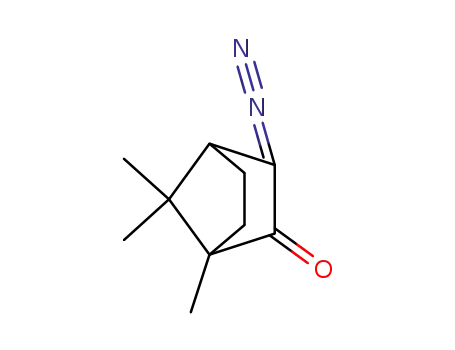
3-diazocamphor
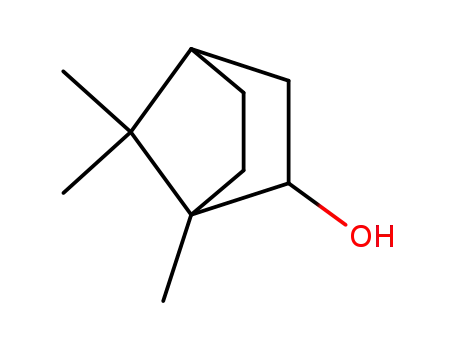
1,7,7-trimethyl bicyclo[2.2.1]heptan-2-ol
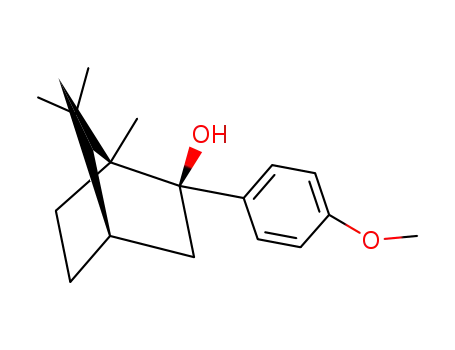
p-Anisylisoborneol
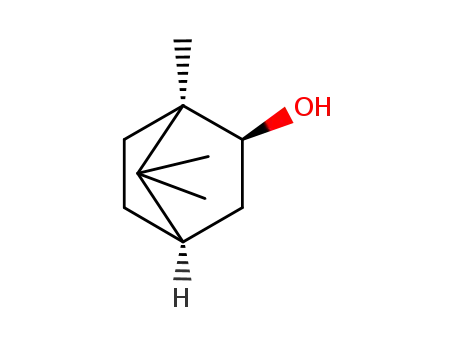
d-borneol
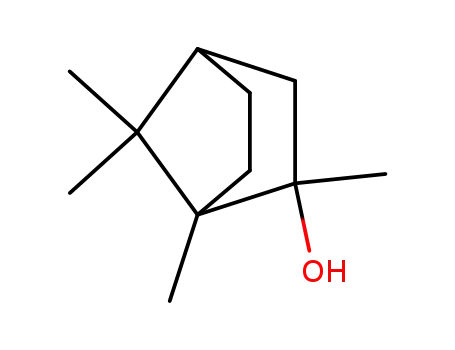
2-methyl-isoborneol
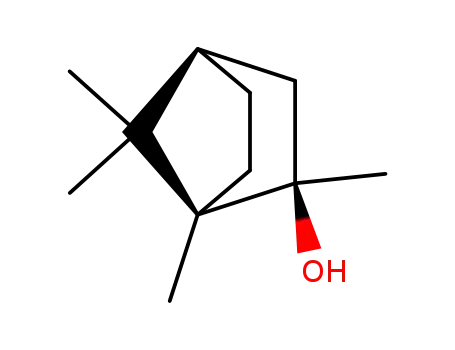
(+)-R-methylisoborneol