Your Location:Home > Products > Organic Raw Materials > Uridine


CasNo: 58-96-8
MF: C9H12N2O6
Appearance: white to off-white crystalline powder
Uridine is an important nucleoside that appears as a white crystalline powder. Uridine plays an important role in various physiological processes in the body, especially in nucleic acid metabolism and genetic information transmission. As a component of RNA, uridine is essential for gene expression and protein synthesis. It can be obtained by biological fermentation or chemical synthesis. In the medical field, uridine and its derivatives have potential applications in the treatment of certain neurological diseases and can be used as auxiliary components of anti-tumor drugs. For example, the mechanism by which uridine plays a role in the treatment of neurodegenerative diseases is a closely watched research topic.
InChI:InChI=1/C9H12N2O6/c12-3-4-6(14)7(15)8(17-4)11-2-1-5(13)10-9(11)16/h1-2,4,6-8,12,14-15H,3H2,(H,10,13,16)/t4-,6+,7-,8-/m0/s1
A study by Hanssen, Rigoux, et al. shows that, in humans, circulating uridine is a mediator of hunger and food intake that is dynamically regulated by caloric ingestion. Oral administration of uridine monophosphate (UMP) increases circulating uridine levels and can, under the right conditions, alter food behavior regulation.
Hydrolytic reactions of the RP and SP di...
While previous studies have shown that uridine can be salvaged to support pyrimidine synthesis in the setting of mitochondrial oxidative phosphorylation deficiency1, our work demonstrates that the ribose moiety of uridine or RNA can be salvaged to fulfil energy requirements via a pathway based on: (1) the phosphorylytic cleavage of uridine by uridine phosphorylase UPP1/UPP2...
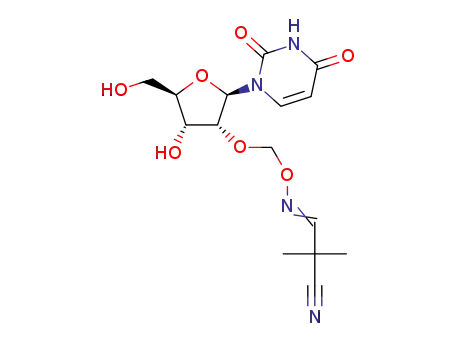
2’-O-(2-cyano-2,2-dimethylethanimine-N-oxymethyl)uridine

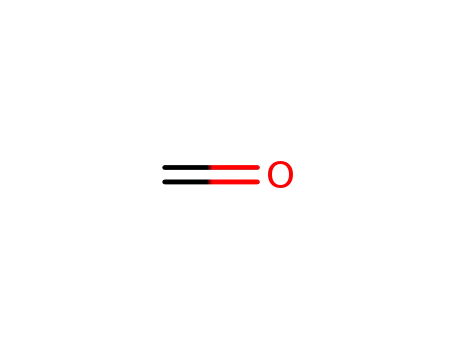
formaldehyd

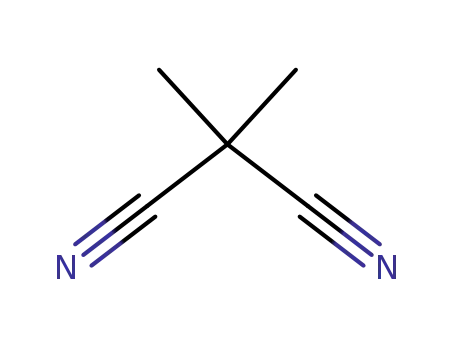
2,2-dimethylmalononitrile

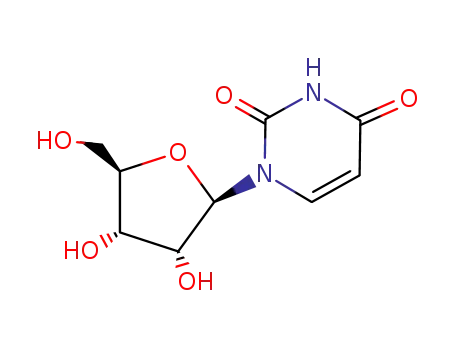
uridine
| Conditions | Yield |
|---|---|
|
With tetrabutyl ammonium fluoride; In dimethyl sulfoxide; at 25 ℃; for 0.25h;
|
5'-CGGCUXUUAACCGA-3', X=2-deoxouridine

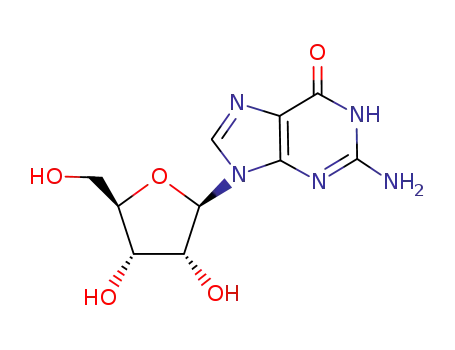
G

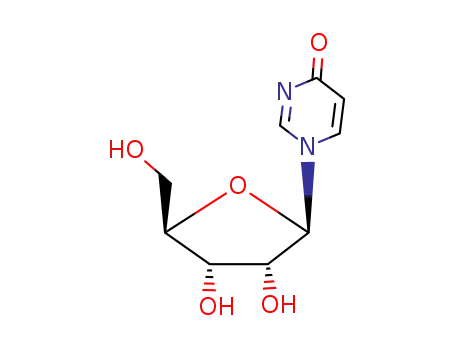
1-(β-D-ribofuranosyl)-4-pyrimidinone


uridine

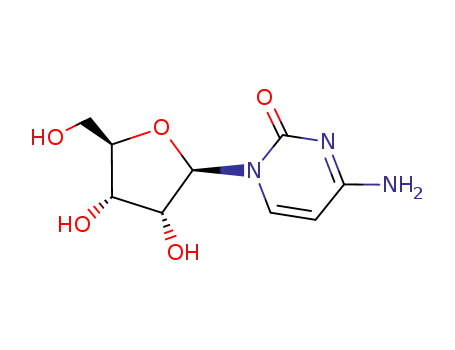
CYTIDINE

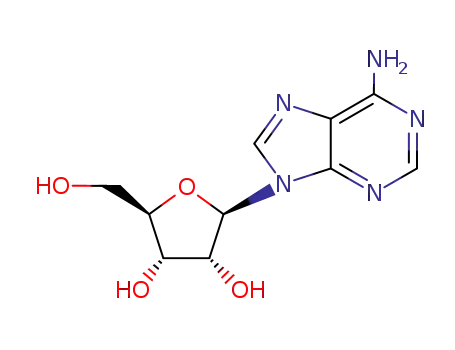
adenosine
| Conditions | Yield |
|---|---|
|
5'-CGGCUXUUAACCGA-3', X=2-deoxouridine; With 1U P1 nuclease; In aq. buffer; at 37 ℃; for 16h; pH=7; Enzymatic reaction;
With 2U alkaline phosphatase; In aq. buffer; at 37 ℃; for 1h; Enzymatic reaction;
|
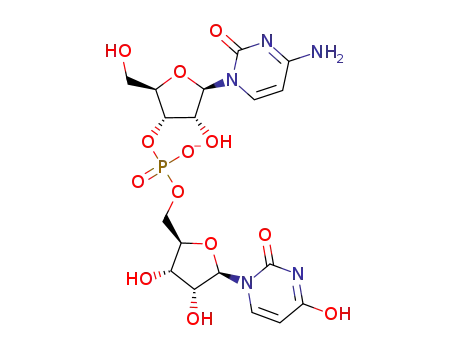
C18H23N5O13P(1-)
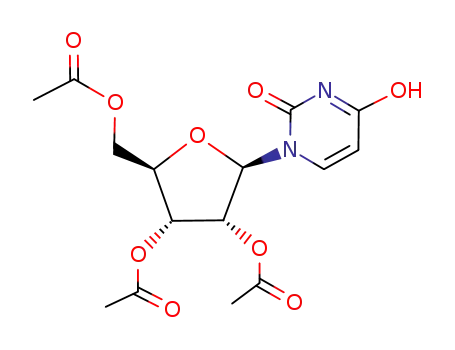
2',3',5'-tri-O-acetyl-uridine
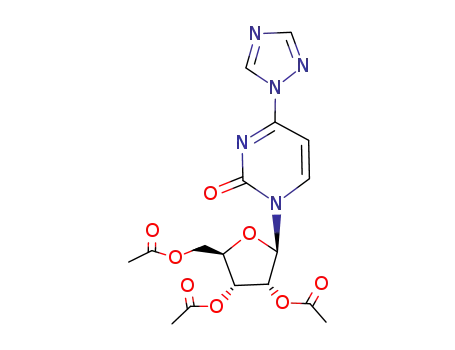
[(2R,3R,4R)-3,4-diacetoxy-5-[2-oxo-4-(1,2,4-triazol-1-yl)pyrimidin-1-yl]tetrahydrofuran-2-yl]methyl acetate
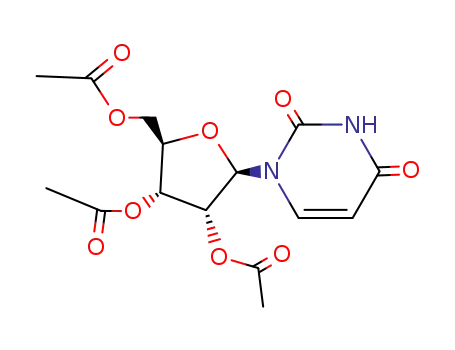
Tri-O-acetyluridine
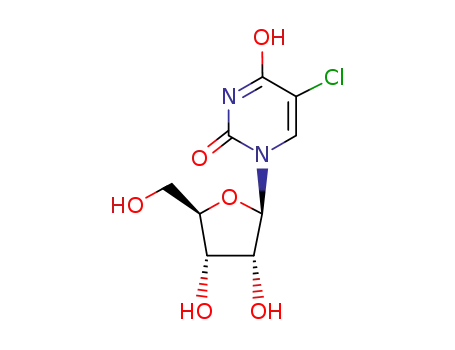
5-chlorouridine
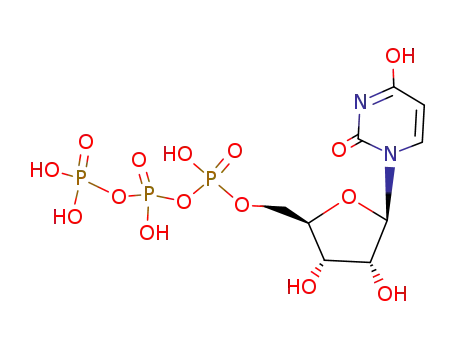
triphosphoric acid 1-uridin-5'-yl ester
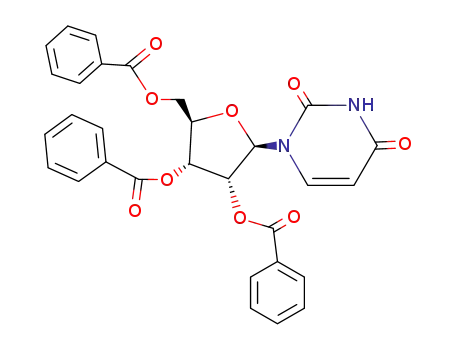
tribenzoyl uridine

Tri-O-acetyluridine