Your Location:Home > Products > Chemical Reagents > Monobenzone


CasNo: 103-16-2
MF: C13H12O2
Appearance: cream to beige-light brownish crystals
Monobenzone is a depigmenting agent. It is commonly used to treat skin hyperpigmentation conditions such as vitiligo. It achieves the depigmentation effect by destroying melanocytes and reducing the production of melanin in the skin. The use of monobenzone requires strict adherence to doctor's orders and specific usage methods, as improper use may lead to uneven depigmentation or other skin problems. It usually comes in the form of a cream or ointment and should be used with caution under the guidance of a doctor. For example, for some severe pigmentation diseases, monobenzone may be an effective treatment option, but the skin's reaction needs to be closely observed during use.
InChI:InChI=1/C14H14O.C6H6O2/c1-3-7-13(8-4-1)11-15-12-14-9-5-2-6-10-14;7-5-1-2-6(8)4-3-5/h1-10H,11-12H2;1-4,7-8H
Efficient conversion of toluenesulfonate...
We investigated a novel immunotherapeutic approach of the skin-depigmenting compound monobenzone synergizing with imiquimod in inducing antimelanoma immunity and melanoma regression. Stage III-IV melanoma patients with non-resectable cutaneous melanoma metastases were treated with monobenzone and imiquimod (MI) therapy applied locally to cutaneous metastases and adjacent skin during 12 weeks, or longer.
A simple and cheap oxidative procedure u...
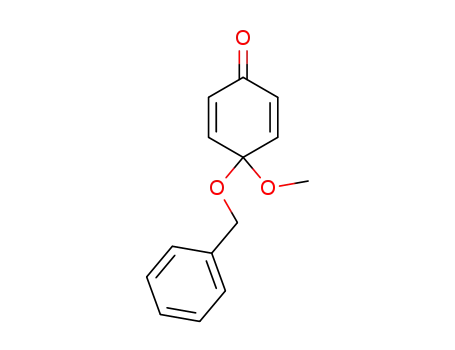
4-(benzyloxy)-4-methoxy-2,5-cyclohexadien-1-one

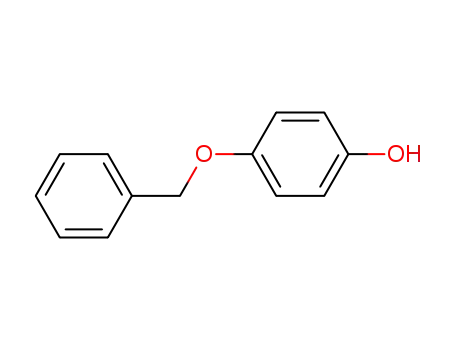
4-Benzyloxyphenol

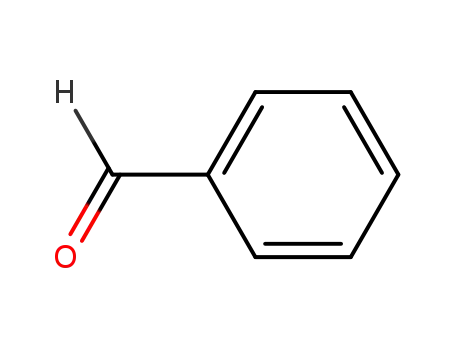
benzaldehyde

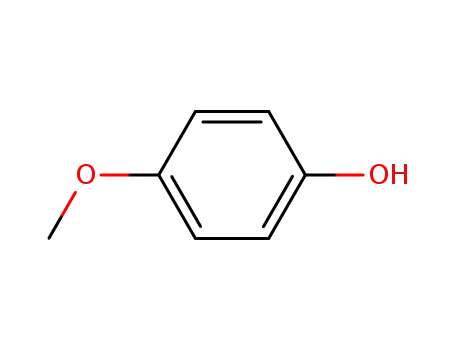
4-methoxy-phenol
| Conditions | Yield |
|---|---|
|
In tetrahydrofuran; at 180 ℃; for 4.5h;
|
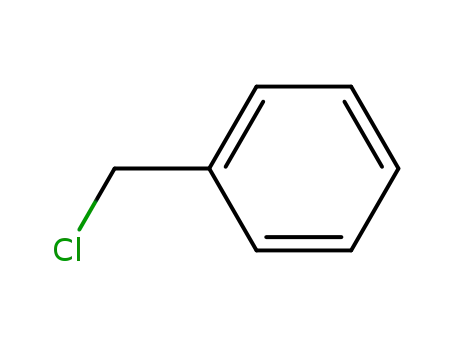
benzyl chloride

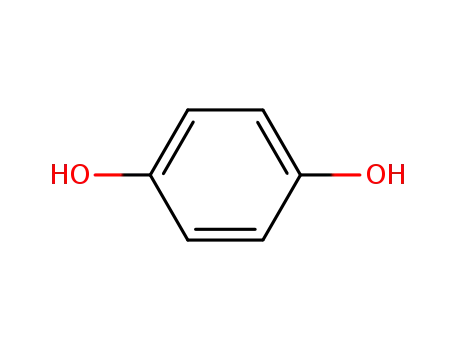
hydroquinone


4-Benzyloxyphenol

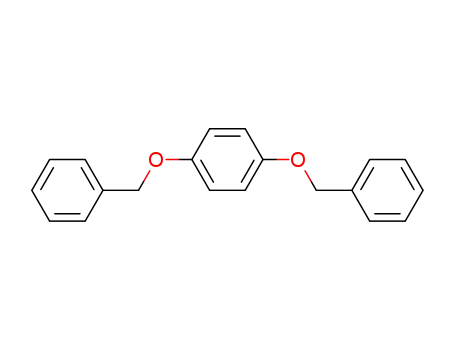
1,4-dibenzyloxybenzene
| Conditions | Yield |
|---|---|
|
With xylene;
|
|
|
With potassium carbonate; acetone;
|
|
|
With ethanol; sodium ethanolate;
|
|
|
With ethanol; anion-exchanger;
|
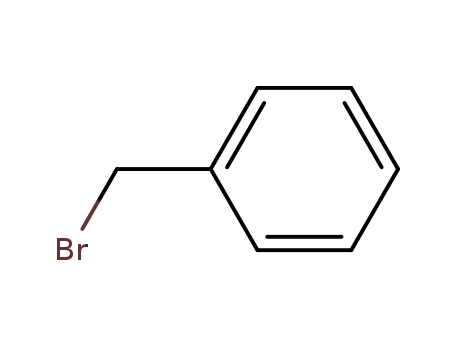
benzyl bromide

hydroquinone
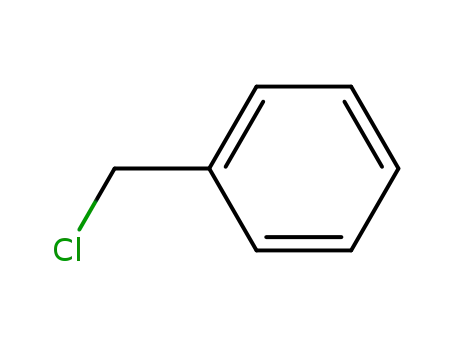
benzyl chloride
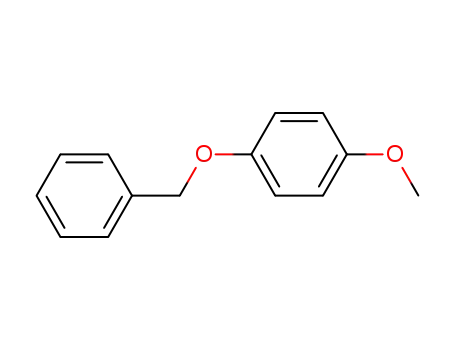
4-methoxyphenyl benzyl ether
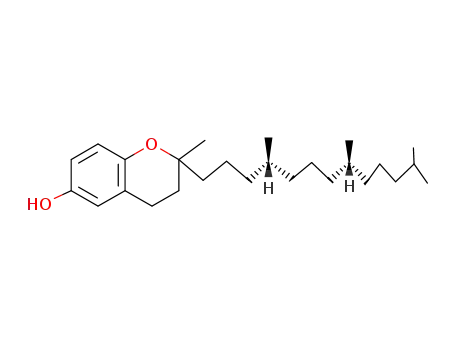
(2RS,4'R,8'R)-Tocol
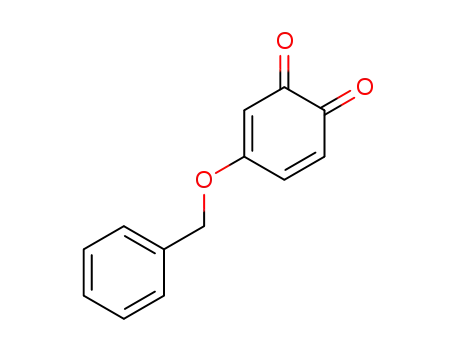
4-benzyloxy-[1,2]benzoquinone
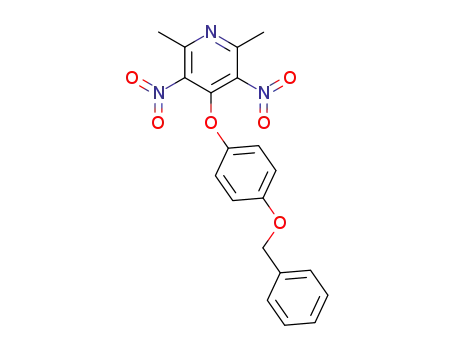
4-(4-benzyloxy-phenoxy)-2,6-dimethyl-3,5-dinitro-pyridine
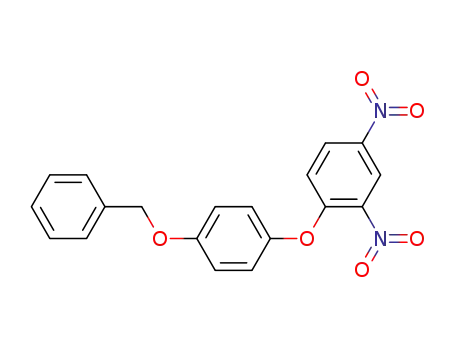
1-benzyloxy-4-(2,4-dinitro-phenoxy)-benzene