Your Location:Home > Products > Chemical Reagents > 4'-Chloropropiophenone


CasNo: 6285-05-8
MF: C9H9ClO
Appearance: crystalline mass
4'-Chloropropionophenone is an organic halogenated ketone compound. In organic synthesis, it is often used as an important intermediate to synthesize a variety of organic compounds with specific functions. For example, it plays a key role in the synthesis of medicines, pesticides and fine chemicals. The presence of its chlorine atom and carbonyl group makes it highly reactive, and it can undergo a variety of substitution, addition and other reactions. However, when operating and using 4'-chloropropionophenone, it is necessary to pay attention to its toxicity and irritation, and take protective measures. 4'-Chloropropionophenone is usually prepared by reacting the corresponding phenone compound with a chlorination reagent. EMEI ELECTRONIC COMMERCE FIRM work for customer satisfaction and achieve the greatest recognition from customers within the scope permitted by law. We focus on every detail and strive to provide customers with procurement, research and development, quality control, Grasp the best service in terms of safe transportation, so as to establish long-term cooperative relationships with customers.
The versatile application of different f...
Fermenting baker's yeast converts 4′-chloropropiophenone 1 and 3′-chloropropiophenone 2 into enantiopure (S)-(−)-1-(4′-chlorophenyl)propan-1-ol (S)-3 and (S)-(+)-1-(3′-chlorophenyl)propan-1-ol (S)-4, respectively. Application of inhibitors and organic solvents as additives enhanced the enantiomeric excesses. Enantiopure compounds (R)-(+)-1-(4′-chlorophenyl)propan-1-ol ((R)-3) and (R)-(+)-1-(3′-chlorophenyl)propan-1-ol (R)-4 were prepared by lipase-mediated esterifications of the racemic alcohols. Maximum inhibition of the growth of the phytopathogenic fungus Botrytis cinerea was shown for the (R)-enantiomers.
Ketones are of great importance in synth...
The synthesis of a range of novel α-sulf...
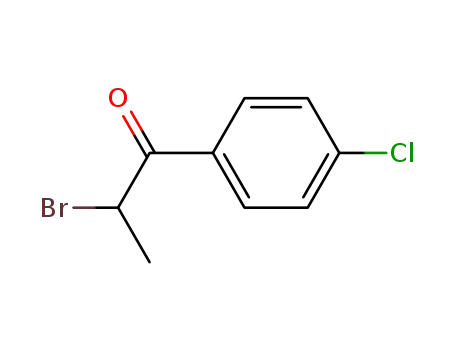
2-bromo-1-(4-chlorophenyl)-1-propanone

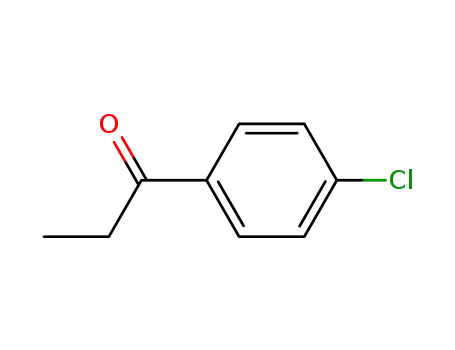
4'-chloropropiophenone

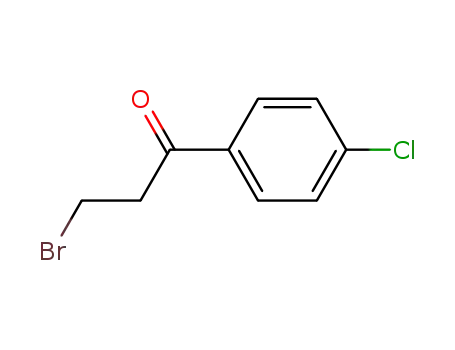
3-bromo-1-(4-chlorophenyl)propan-1-one
| Conditions | Yield |
|---|---|
|
In acetonitrile; Photolysis;
|
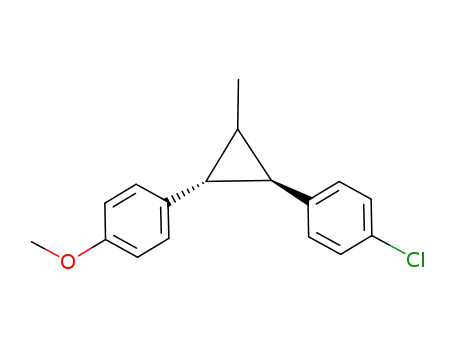
1-(4-Chlorophenyl)-2-(4-methoxyphenyl)-3-methylcyclopropane


4'-chloropropiophenone

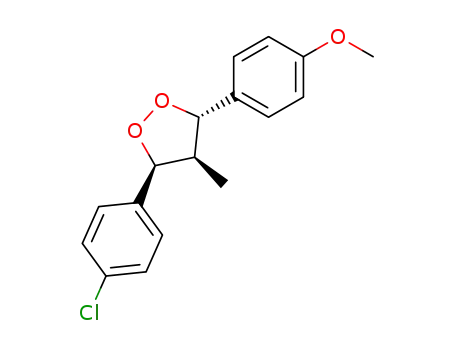
r-3-(4-Chlorophenyl)-c-4-methyl-t-5-(4-methoxyphenyl)-1,2-dioxolane

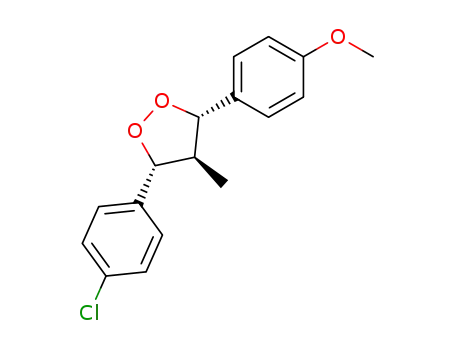
r-3-(4-Chlorophenyl)-t-4-methyl-c-5-(4-methoxyphenyl)-1,2-dioxolane

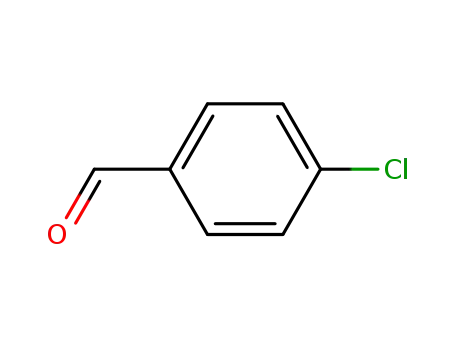
4-chlorobenzaldehyde

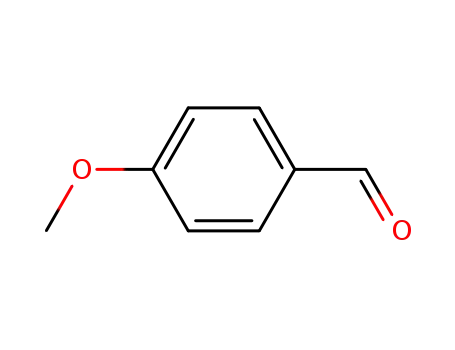
4-methoxy-benzaldehyde

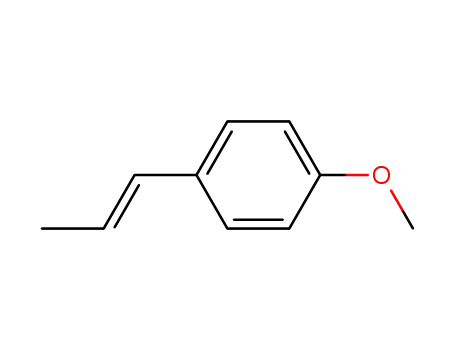
E-1-(4'-methoxyphenyl)prop-1-ene
| Conditions | Yield |
|---|---|
|
With magnesium(II) perchlorate; oxygen; 9,10-Dicyanoanthracene; In acetonitrile; for 0.0333333h; Rate constant; Thermodynamic data; Mechanism; Ambient temperature; Irradiation; ΔG;
|
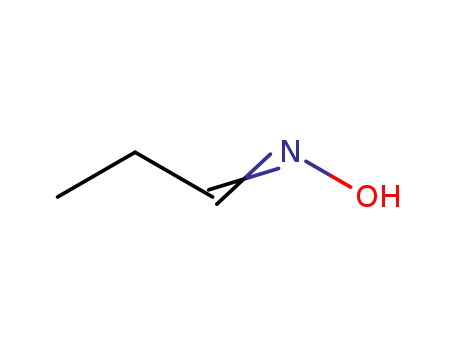
propionaldehyde oxime
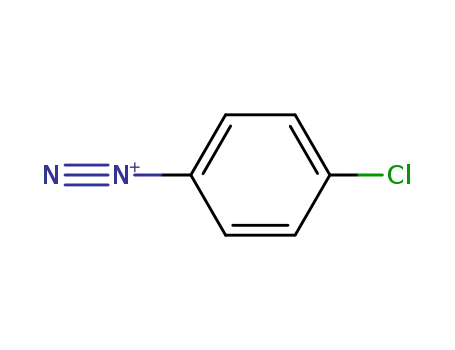
4-chlorophenyldiazonium salt
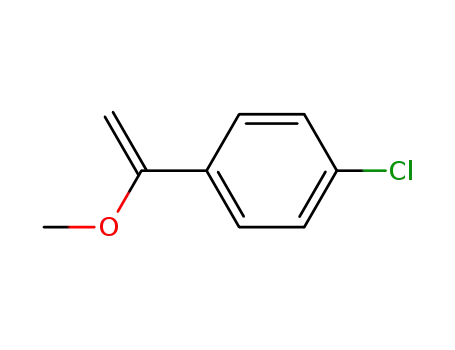
1-(p-chlorophenyl)-1-methoxyethene
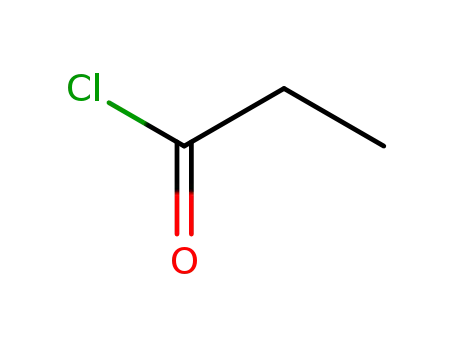
propionyl chloride
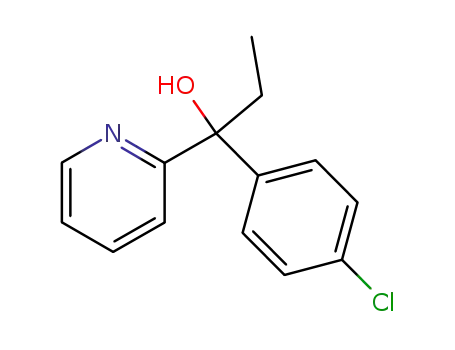
1-(4-chlorophenyl)-1-(2-pyridyl)-1-propanol
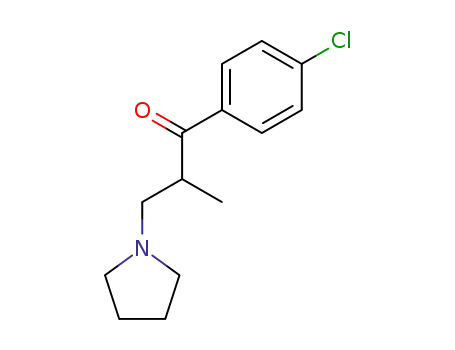
1-(4-chloro-phenyl)-2-methyl-3-pyrrolidino-propan-1-one
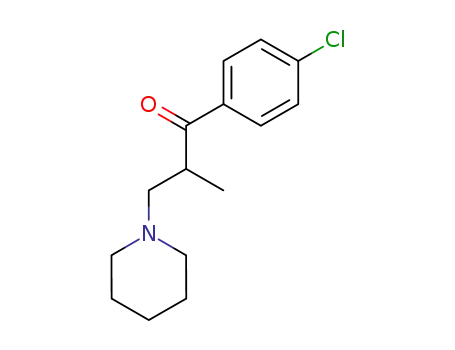
1-(4-chloro-phenyl)-2-methyl-3-piperidino-propan-1-one
para-chlorobenzoic acid