Your Location:Home > Products > Organic Raw Materials > 4-Nitroimidazole


CasNo: 3034-38-6
MF: C3H3N3O2
Appearance: white to light yellow powder
|
Definition |
4-Nitroimidazole is a C-nitro compound that is imidazole bearing a nitro substituent at position 4. It is a member of imidazoles and a C-nitro compound. |
|
General Description |
4-Nitroimidazole is an intermediate during the synthesis of 1-methyl-2,4,5-trinitro imidazole. |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C3H3N3O2/c7-6(8)3-1-4-2-5-3/h1-2H,(H,4,5)
In the crystal structures of the two imidazole derivatives 5-chloro-1,2-dimethyl-4-nitro-1H-imidazole, C5H6ClN3O2, (I), and 2-chloro-1-methyl-4-nitro-1H-imidazole, C4H4ClN3O2, (II), C-Cl...O halogen bonds are the principal specific interactions responsible for the crystal packing. Two different halogen-bond modes are observed: in (I), there is one very short and directional C-Cl...O contact [Cl...O = 2.899 (1) Å], while in (II), the C-Cl group approaches two different O atoms from two different molecules, and the contacts are longer [3.285 (2) and 3.498 (2) Å] and less directional. In (I), relatively short C-H...O hydrogen bonds provide the secondary interactions for building the crystal structure; in (II), the C-H...O contacts are longer but there is a relatively short [pi]-[pi] contact between molecules related by a centre of symmetry. The molecule of (I) is almost planar, the plane of the nitro group making a dihedral angle of 6.97 (7)° with the mean plane of the imidazole ring. The molecule of (II) has crystallographically imposed mirror symmetry and the nitro group lies in the mirror plane.
In an effort to synthesize more effectiv...
Ferrocenylalkyl nitro-imidazoles (4a-h, ...
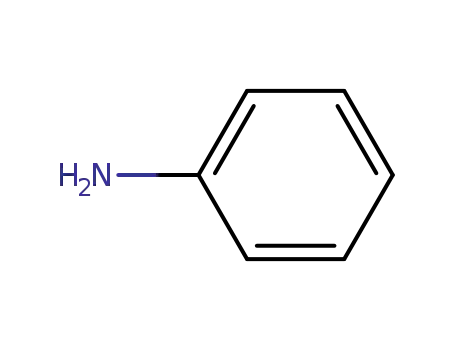
aniline

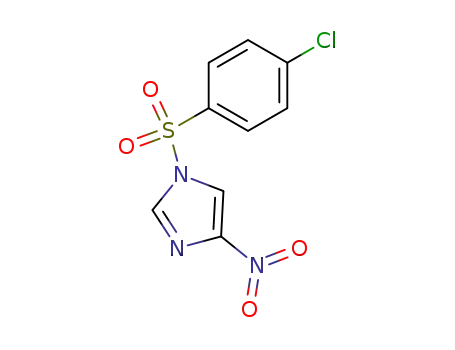
1-(p-chlorobenzenesulfonyl)-4-nitroimidazole

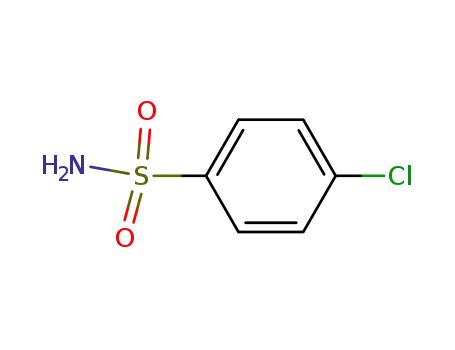
4-Chlorobenzenesulfonamide

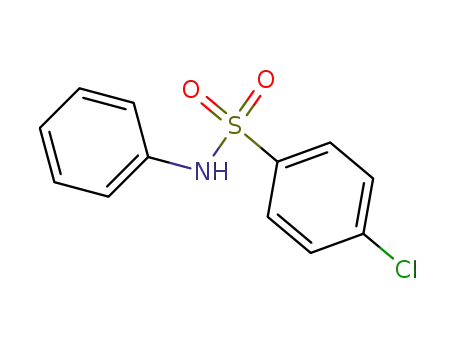
4-chloro-N-phenyl-benzenesulfonamide

1H-4(5)-nitroimidazole

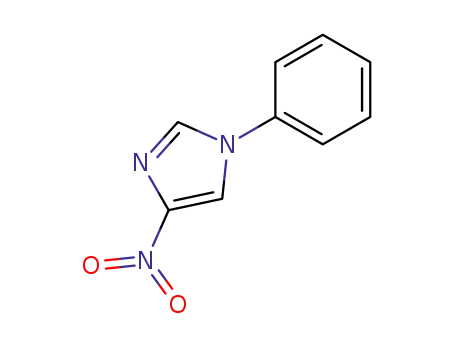
1-phenyl-4-nitro-1H-imidazole
| Conditions | Yield |
|---|---|
|
In methanol; water; Product distribution; Mechanism; 65 deg C to 70 deg C, 2 h, 25 deg C, 1 d; different azolides, anilines, amine, reagent, solvent, reaction time and temperature;
|
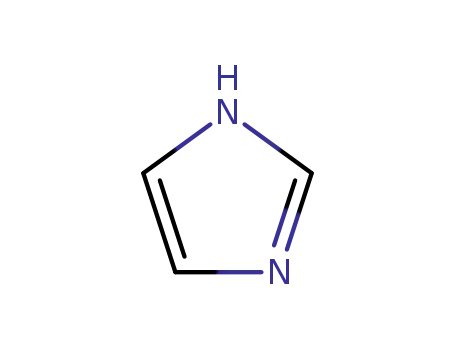
1H-imidazole

1H-4(5)-nitroimidazole
| Conditions | Yield |
|---|---|
|
With sulfuric acid; nitric acid; at 60 ℃; Temperature; Flow reactor;
|
96.1% |
|
With sodium nitrate; In dichloromethane; for 1.5h; Reflux;
|
87% |
|
With nitric acid; sulfuric acid; at 70 - 100 ℃;
|
78.2% |
|
With sodium nitrate; sulfuric acid; at 145 ℃; for 6h;
|
75% |
|
With 1-nitro-1H-pyrazole; sulfuric acid; at 20 - 65 ℃; for 6h;
|
72.4% |
|
With sulfuric acid; nitric acid; at 70 - 100 ℃; for 5h;
|
67% |
|
With sulfuric acid; nitric acid; at 40 - 45 ℃;
|
56% |
|
With sulfuric acid; nitric acid; at 125 ℃; for 8h;
|
46% |
|
With sulfuric acid; nitric acid; at 100 ℃; for 6h; Cooling;
|
46% |
|
With sulfuric acid; nitric acid;
|
|
|
With sulfuric acid; nitric acid;
|
|
|
With sulfuric acid; nitric acid; Heating;
|
|
|
1H-imidazole; With sulfuric acid; at 20 ℃; for 0.5h;
With sulfuric acid; nitric acid; at 55 ℃; for 3h;
|
|
|
With sulfuric acid; nitric acid; at 65 - 120 ℃; for 2.5h; Temperature; Cooling with ice;
|

1H-imidazole
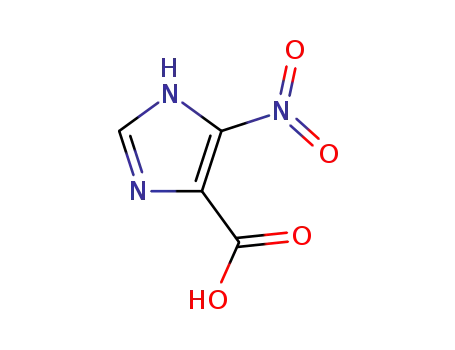
5(4)-nitro-1(3)H-imidazole-4(5)-carboxylic acid
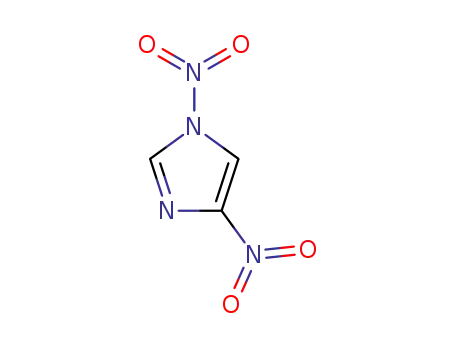
1,4-dinitro-1H-imidazole

aniline
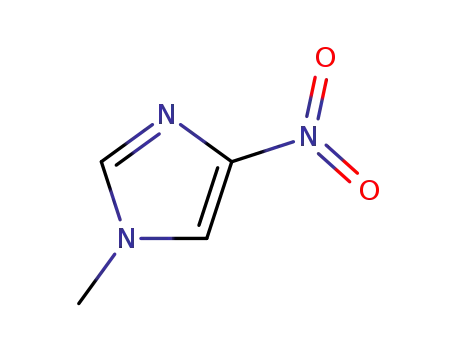
1-methyl-4-nitro-1H-imidazole
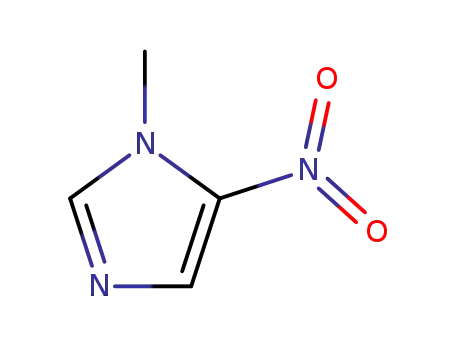
1-methyl-5-nitro-1H-imidazole
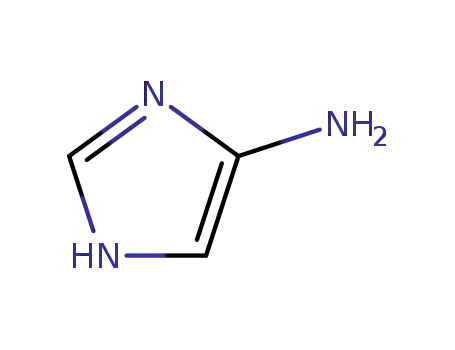
4-amino-1H-imidazole
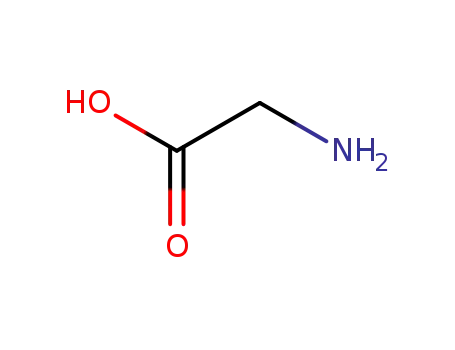
glycine