Your Location:Home > Products > Pharmaceutical Raw Material > Metronidazole


CasNo: 443-48-1
MF: C6H9N3O3
Appearance: white to slightly yellow crystalline powder
|
Description |
Metronidazole (MTZ, 1-[2-hydroxyethyl]-2-methyl-5-nitroimidazole), an antiparasitic and antibacterial compound, is one of the world’s most used drugs. |
|
Brand Name(s) |
Flagyl and generic |
|
Usage |
Metronidazole is used to treat infections of the reproductive system, gastrointestinal (GI) tract, skin, heart, bone, joint, lung, blood, nervous system, and other areas of the body. It is also used to treat certain sexually transmitted diseases (STDs). Metronidazole is a nitroimidazole antibiotic, also known as metronidazol and novonidazol. It was initially used to treat vaginal trichomaniasis, with very significant clinical effects. It is broadly used to prevent and treat oral anaerobic infections. In hospitals, it has been used frequently to prevent and treat respiratory, gastrointestinal, peritoneal, pelvic, skin, soft tissue, joint, and brain infections, cardiomyitis, and septicemia caused by anaerobic bacteria. |
|
Mechanisms of Action |
Metronidazole kills anaerobic microorganisms, and its metabolites in the body during reduction also inhibit them by inhibiting DNA synthesis, thus interfering with bacterial growth and propagation, eventually killing them. Anaerobic bacteria affected include: Bacteroides fragilis, Fusobacterium (so named because of its sharp fusiform shape at both ends,) Clostridium tetani, Peptostreptococcus, and Giardia lamblia. Its mechanism of action in the treatment of parasites is to disrupt protozoans’ nitrogen chains by inhibiting their redox reactions. In vitro experiments have shown that at concentrations of 1-2 mg/L, morphological changes occurred in dissolved amoeba starting at 6-20 hours, killing them all within 24 hours. At a concentration of 0.2 mg/L, dissolved bacteria were killed within 72 hours. |
|
Warnings and Precautions |
Interactions with nitroimidazole antibiotics, ethanol, and nicotine interfere with the oxidation of ethanol and can cause disulfiram reactions, causing symptoms like faster heart rate and decreased blood pressure, so patients should avoid contact with alcohol and smoke less during treatment in order to prevent the occurrence of adverse reactions. |
|
Methods of production |
It is synthetized by 2-methyl-5-nitro imidazole (see 25010) and ethylene oxide addition. 2-methyl-5-nitro imidazole dissolved in formic acid and at 30-40℃ successive adding epoxy ethane, and sulfuric acid in the middle of adding feeding. and reaction for 1 h, after that. Decompression to recycle formic acid, water solution is cooled to 10 ℃, filter. The filtrate with sodium hydroxide solution to adjust pH = 10. Set aside to cool, filtering, washing to nearly alterations into neutral, recrystallization in water. Activated carbon decolorization to get metronidazole. |
|
Pharmacology and mechanism of action |
Metronidazole is a 5-nitroimidazole derivative which was originally introduced against Trichomonas vaginalis in 1960. Soon it was shown to possess a broad spectrum of activity against other protozoal infections such as amoebiasis and giardiasis, and more recently against infections due to anaerobic bacteria [1]. The mechanism of action of metronidazole is not well understood. In the parasite, the 5-nitro group of the drug undergoes reductive transformation to a cytotoxic intermediate which binds to the helical structure of the DNA leading to strand breakage and eventual cell death [2]. |
|
Indications |
Against infections caused by Trichomonas vaginalis, Entamoeba histolytica (acute intestinal type and liver abscesses), Giardia lamblia and Dracunculus medinensis. During treatment of trichomoniasis it is wise to treat the male partner as well. In amoebiasis, a luminal amoebicide is added to eliminate surviving organisms in the colon. Metronidazole is also used for the treatment of infections due to anaerobic bacteria. |
|
Side effects |
Side effects with doses used to treat protozoal infections are usually mild, reversible and self-limiting and may affect 4% to 5% of treated patients. The most common are gastrointestinal disturbances (nausea, vomiting, epigastric pain, metallic taste, furring of the tongue), intolerance to alcohol (disulfiram-like effect) and central nervous system effects (headache, dizziness and sleepiness) [3]. Other side effects reported include urticaria, darkening of the urine with a reddish-brown discoloration and transient neutropenia [4]. During prolonged high doses, the drug may cause severe neurotoxic side effects such as peripheral neuropathy, paraesthesia and epileptiform seizures [3,4]. Few case reports of bone marrow depression [5], gynecomastia [6] and acute pancreatitis [7] have been reported. Although metronidazole is mutagenic in bacteria and carcinogenic in rodents, no association with human cancer has been proven . |
|
Contraindications and precautions |
Dosage reductions should be made in patients with severe hepatic failure. Because of its potential neurotoxicity and neutropenia the drug should be given with caution to patients with diseases of the CNS or with a history of blood dyscrasia. Patients should be warned of a disulfiram-like reaction if the drug is taken together with alcohol. Metronidazole should be used with extra caution in patients being treated with warfarin (see interactions). |
|
Interactions |
Metronidazole is a weak inhibitor of alcohol dehydrogenase. Simultaneous administration of metronidazole and disulfiram has been reported to cause an acute psychosis or mental confusion. This effect was observed in 6 of 29 chronic alcoholic men given both drugs, but in none of those given placebo plus disulfiram [8]. Metronidazole inhibits the ring oxidation of S (+) warfarin and significant bleeding can occur if the two drugs are taken together [9]. Significant increase of hepatic clearance of metronidazole has been reported when the drug was taken together with phenobarbital [10, 11] or prednisone [11]. |
|
Preparations |
Metronidazole, 2-methyl-5-nitroimidazol-1-ethanol (37.2. 10), is made by nitrating 2-methylimidazole to make 2-methyl-5-nitroimidazole (37.2. 9), which is then reacted with 2-chloroethanol or ethylenoxide, which is easily transformed to the desired metronidazole. |
|
Therapeutic Function |
Antiprotozoal |
|
Antimicrobial activity |
Metronidazole inhibits E. histolytica, G. lamblia, T. vaginalis, Blastocystis hominis, B. coli, and the helminth Dracunculus medinensis. It is also bactericidal for obligate anaerobic gram-positive and gram-negative bacteria except Actinomyces spp. It is not active against aerobes or facultative anaerobes. Drug resistance is infrequent; the mechanism of resistance is not understood. Tinidazole, a 5-nitroimidazole closely related to metronidazole, is effective against vaginal trichomoniasis resistant to metronidazole. |
|
Acquired resistance |
Although resistance in Bacteroides spp. and T. vaginalis is well documented, it is uncommon. Resistance occurs more frequently in H. pylori and failure of treatment with triple drug regimens may be associated with resistance to the metronidazole component. |
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
Metronidazole darkens on exposure to light. Metronidazole is incompatible with strong oxidizing agents. . |
|
Fire Hazard |
Flash point data for Metronidazole are not available; however, Metronidazole is probably combustible. |
|
Pharmacology |
Absorption from the intestinal tract is usually good. Food delays but does not reduce absorption.The drug is distributed in body fluids and has a half-life of about 8 hours. High levels are found in plasma and cerebrospinal fluid (CSF). Less than 20% binds to plasma proteins. Metronidazole is metabolized by oxidation and glucuronide formation in the liver and is primarily excreted by the kidneys, although small amounts can be found in saliva and breast milk. Dose reduction is generally unnecessary in renal failure. |
|
Safety Profile |
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. Moderately toxic by ingestion, intraperitoneal, and subcutaneous routes. Human systemic effects by ingestion: paresthesia, nerve or sheath structural changes, eye changes, tremors, fever, jaundice and other liver changes, hearingacuity changes, somnolence, and ataxia. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits toxic fumes of NOx. |
|
Synthesis |
Metronidazole, 2-methyl-5-nitroimidazol-1-ethanol (37.2.10), is made by nitrating 2-methylimidazole to make 2-methyl-5-nitroimidazole (37.2.9), which is then reacted with 2-chloroethanol or ethylenoxide, which is easily transformed to the desired metronidazole. |
|
Potential Exposure |
Metronidazole is an orally administered drug for the treatment of infections due to entamoeba histolytica; trichomonas vaginalis; giardia lamblia, and has also been used for treating Vincent’s infection. It can be used as a trichomonacide in veterinary medicine. One firm has petitioned EPA to use metronidazole as a disinfectant for cooling tower water. |
|
Veterinary Drugs and Treatments |
Although there are no veterinary-approved metronidazole products, the drug has been used extensively in the treatment of Giardia in both dogs and cats. It is also used clinically in small animals for the treatment of other parasites (Trichomonas and Balantidium coli) as well as treating both enteric and systemic anaerobic infections. In horses, metronidazole has been used clinically for the treatment of anaerobic infections. |
|
Drug interactions |
Potentially hazardous interactions with other drugs Alcohol: disulfiram-like reaction. Anticoagulants: effects of coumarins enhanced. Antiepileptics: metabolism of phenytoin inhibited; concentration reduced by phenobarbital. Busulfan: concentration of busulfan increased - risk of toxicity. Ciclosporin: raised blood level of ciclosporin. Cytotoxics: busulfan concentration increased; metabolism of fluorouracil inhibited. |
|
Carcinogenicity |
Metronidazole is reasonably anticipated to be a human carcinogenbased on sufficient evidence of carcinogenicity from studies in experimental animals. |
|
Environmental Fate |
Due to metronidazole’s use as a pesticide, it may have been directly released into the environment. It lacks an adequate chromophore for absorbing light and undergoing photolytic degradation. In addition, in vitro assays demonstrated the compound’s robust stability in the atmosphere or aqueous environments. Metronidazole exhibited a soil half-life between 10 and 27 days. |
|
Metabolism |
Metronidazole is available in a variety of dosage forms, including IV, oral, rectal, and vaginal suppositories. The bioavailability of metronidazole is nearly 100% when administered orally but is significantly less when administered via the rectal route (67–82%) or the vaginal route (19–56%). The drug is not bound to plasma protein. Distribution of the drug is fairly uniform through out the body, including mother's milk. Liver metabolism of metronidazole leads to two major metabolites: hydroxylation of the 2-methyl group to 2-hydroxymethylmetronidazole (HM), and oxidation to metronidazole acetic acid. Both compounds possess biological activity. Additionally, HM is found in the urine as glucuronide and sulfate conjugates. In addition, a small amount of metronidazole is oxidized to acetamide, a known carcinogen in rats but not in humans. |
|
Toxicity evaluation |
Metronidazole is a prodrug that requires reductive activation of the nitro group by susceptible organisms. The reduction causes nitro radical formation and destruction of the organism’s DNA. The mechanism of neurotoxicity is thought to be due to axonal degeneration. Metronidazole has been shown to bind neuronal RNA in rodent models, thus inhibiting protein synthesis and causing degeneration. Metronidazole is also capable of producing a disulfiram-type reaction with ethanol ingestion. This reaction is hypothesized to occur due to metronidazole inhibition of aldehyde dehydrogenase. |
|
Incompatibilities |
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. |
|
Waste Disposal |
Dispose of contents and container to an approved waste disposal plant. All federal, state, and local environmental regulations must be observed. It is inappropriate and possibly dangerous to the environment to dispose of expired or waste drugs and pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, doublebagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged, and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator. |
|
Who Evaluation |
Evaluation year: 1989 |
InChI:InChI=1/C6H9N3O3/c1-5-7-6(9(11)12)4-8(5)2-3-10/h4,10H,2-3H2,1H3
In this report, the short-term results of metronidazole treatment plus mechanical debridement in patients with extensive periodontal disease and of a double-blind clinical study in which metronidazole plus mechanical debridement is compared to placebo plus mechanical debridement are described. The findings indicate that 1 week of systemic metronidazole can optimize the clinical reduction of pockets and increase the apparent attachment in periodontitis patients who receive concurrent mechanical debridement of their root surfaces.
This article discusses the ability of metronidazole to improve periodontal health. Review of the drug's pharmacology and potential side effects indicate that it poses little threat to humans …
The invention discloses a method for syn...
The invention discloses a synthetic meth...
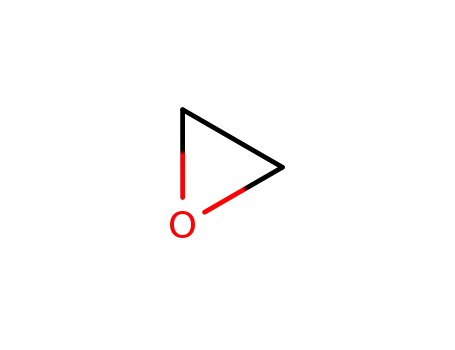
oxirane
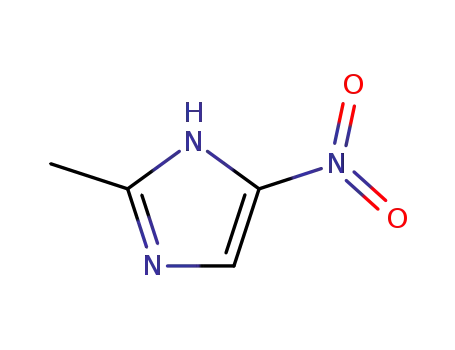
2-methyl-5-nitro-1H-imidazole
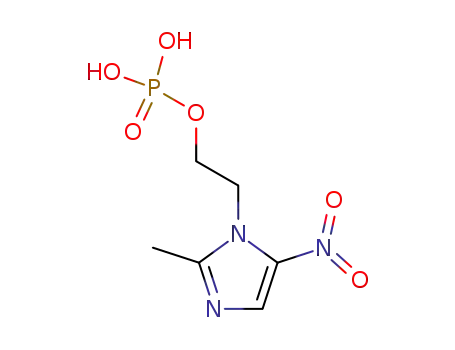
metronidazole phosphate
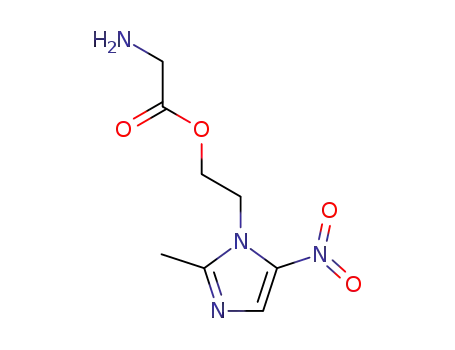
glycine ester of metronidaxole
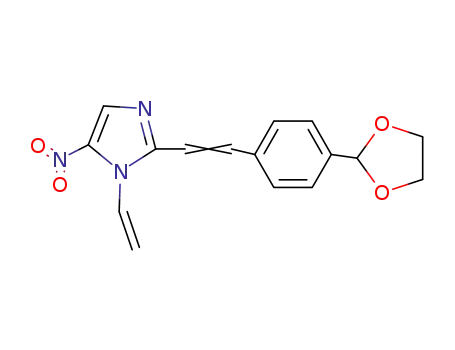
2-(4-[1,3]dioxolan-2-yl-styryl)-5-nitro-1-vinyl-1H-imidazole
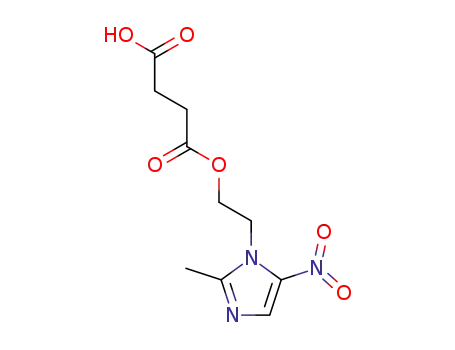
4-[2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethoxy]-4-oxobutanoic acid
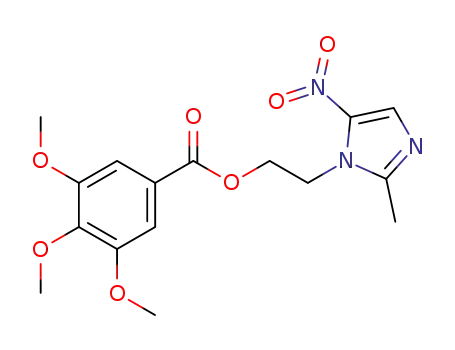
3,4,5-trimethoxy-benzoic acid 2-(2-methyl-5-nitro-imidazol-1-yl)-ethyl ester
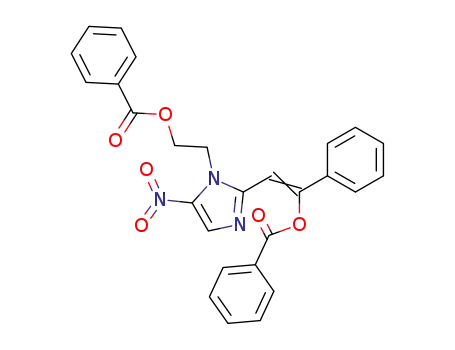
1-(2-Benzoyloxyethyl)-5-nitro-2-imidazole-α-phenylethenol benzoate