Your Location:Home > Products > Organic Raw Materials > Octyl 4-methoxycinnamate


CasNo: 5466-77-3
MF: C18H26O3
Appearance: colourless or pale yellow liquid
Octyl 4-methoxycinnamate is a colorless to light yellow oily liquid. It is a common UV absorber and is widely used in cosmetics and sunscreen products. Octyl 4-methoxycinnamate can effectively absorb UVA radiation, reduce UV damage to the skin, and prevent skin tanning and sunburn. It is usually prepared by chemical synthesis. In the field of cosmetics, it is an important ingredient in many sunscreens, lotions and creams. For example, in summer outdoor activities, using sunscreen products containing octyl 4-methoxycinnamate can reduce the risk of skin damage by UV rays and keep the skin healthy.
InChI:InChI=1/C18H26O3/c1-4-6-7-8-16(5-2)21-18(19)14-11-15-9-12-17(20-3)13-10-15/h9-14,16H,4-8H2,1-3H3/b14-11+
Olefin metathesis has been widely explor...
Reactions of sunscreen agents, octyl dimethyl-p-aminobenzoate (ODPABA) and octyl-p-methoxycinnamate (OMC), with hypochlorite in aqueous solution were investigated under the conditions that simulate swimming pool disinfection sites.
A series of new unsymmetrical (XYC–1 typ...
We report for the first time cyclic phos...
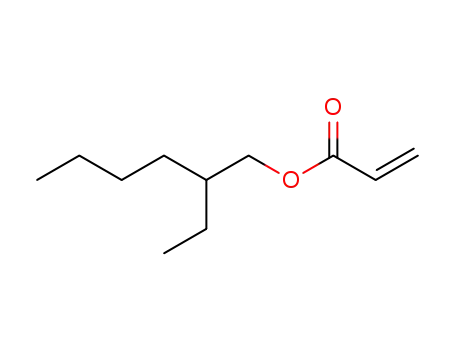
2-Ethylhexyl acrylate

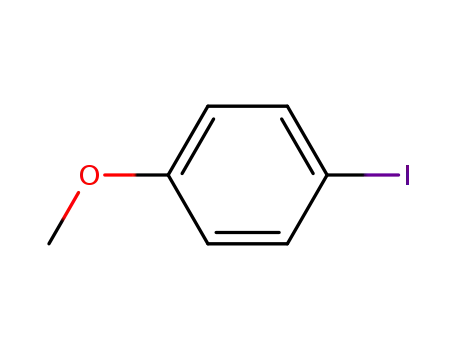
para-iodoanisole

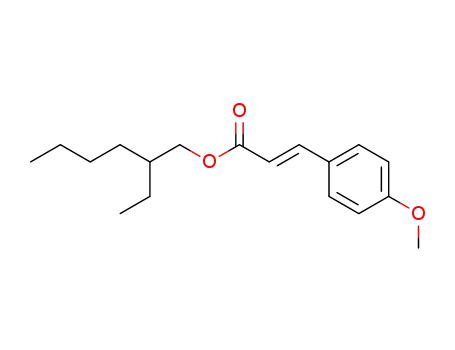
2-ethylhexyl methoxycinnamate
| Conditions | Yield |
|---|---|
|
With tetrabutylammomium bromide; C32H26Cl2N8O4Pd2; potassium carbonate; In methanol; water; for 0.416667h; Reflux;
|
97% |
|
With C43H54NO5P; palladium diacetate; triethylamine; In water; at 40 ℃; for 12h;
|
95% |
|
With tributyl-amine; chloro-[2-(9-phenyl-1,10-phenanthrolin-2-yl)phenyl]palladium; In 1-methyl-pyrrolidin-2-one; at 140 ℃; for 15h; Inert atmosphere;
|
94% |
|
With (Bis(tri-tert-butylphosphine)palladium(0)); SPGS-550-M; NOK; triethylamine; In water; for 5h; Reagent/catalyst; Inert atmosphere; Microwave irradiation; Sealed tube;
|
93% |
|
With triethylamine; 4,4'-dichlorobenzophenone oxime derived palladacycle; In 1-methyl-pyrrolidin-2-one; at 110 ℃; for 22h;
|
85% |
|
With dichloro[1,1'-bis(di-t-butylphosphino)ferrocene]palladium(II); pyridinium p-toluenesulfonate; triethylamine; In water; at 20 ℃; under 760.051 Torr; Inert atmosphere; Micellar solution;
|
84% |
|
With 1-methyl-pyrrolidin-2-one; triethylamine; polymer-bound Pd(0) phosphine catalyst; at 90 ℃;
|
|
|
With acetic acid; diisopropylamine; palladium on charcoal; In toluene;
|
|
|
With potassium acetate; In 1-methyl-pyrrolidin-2-one; at 135 ℃; for 24h;
|
60 %Chromat. |
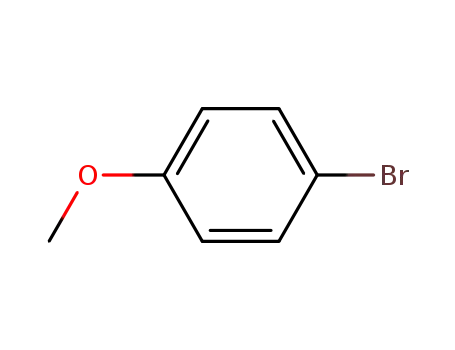
1-bromo-4-methoxy-benzene


2-Ethylhexyl acrylate


2-ethylhexyl methoxycinnamate
| Conditions | Yield |
|---|---|
|
With tris(dibenzylideneacetone)dipalladium (0); tri-tert-butyl phosphine; Cy2NMe2; In 1,4-dioxane; at 20 ℃; for 148h;
|
83% |
|
With C21H21ClN4Pd; triethylamine; In 1-methyl-pyrrolidin-2-one; at 140 ℃; for 8h;
|
73% |
|
With sodium acetate;
|
71% |
|
With tri-n-propylamine; In 1-methyl-pyrrolidin-2-one; at 175 ℃; for 24h; Inert atmosphere;
|
34% |
|
With dimethylaminoacetic acid; bis(benzonitrile)palladium(II) dichloride;
|

1-bromo-4-methoxy-benzene

2-Ethylhexyl acrylate
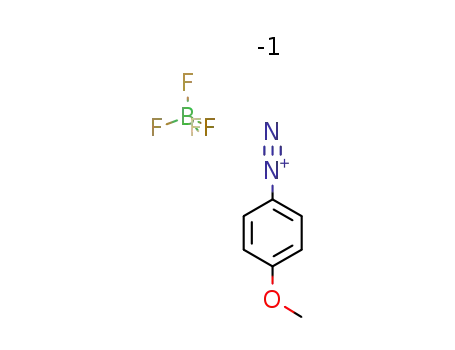
4-methoxybenzenediazonium tetrafluoroborate
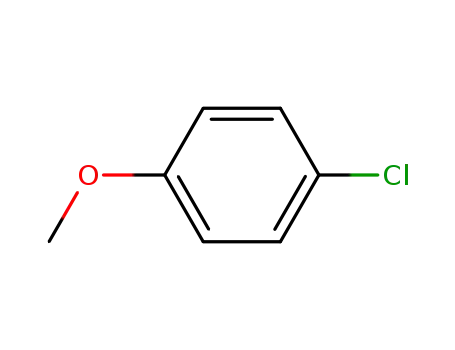
4-chloromethoxybenzene
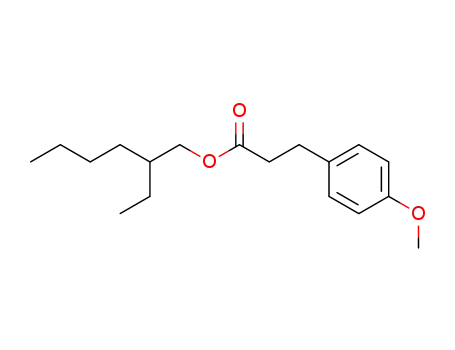
3-(4-methoxy-phenyl)-propionic acid 2-ethyl-hexyl ester
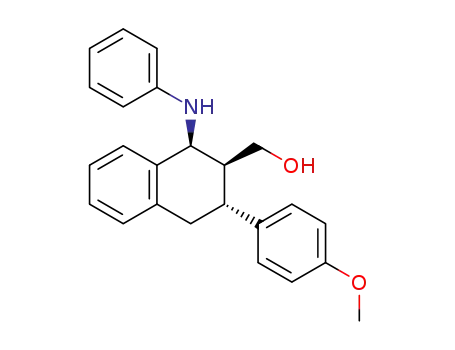
2-hydroxymethyl-3-(p-methoxyphenyl)-1-phenylamino-1,2,3,4-tetrahydronaphthalene
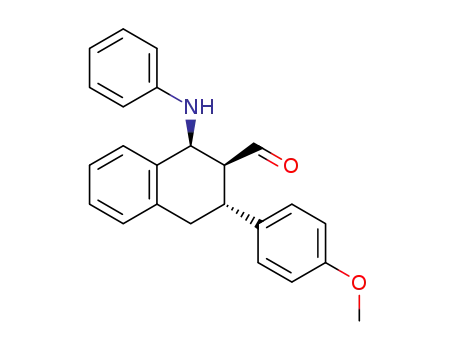
C24H23NO2
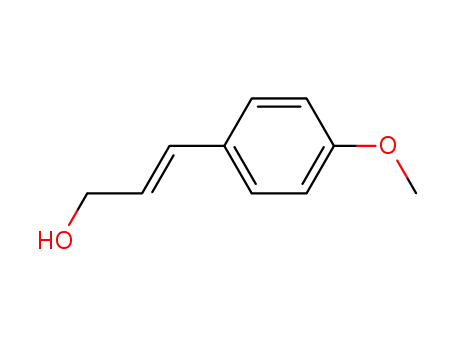
(E)-p-methoxy-cinnamyl alcohol